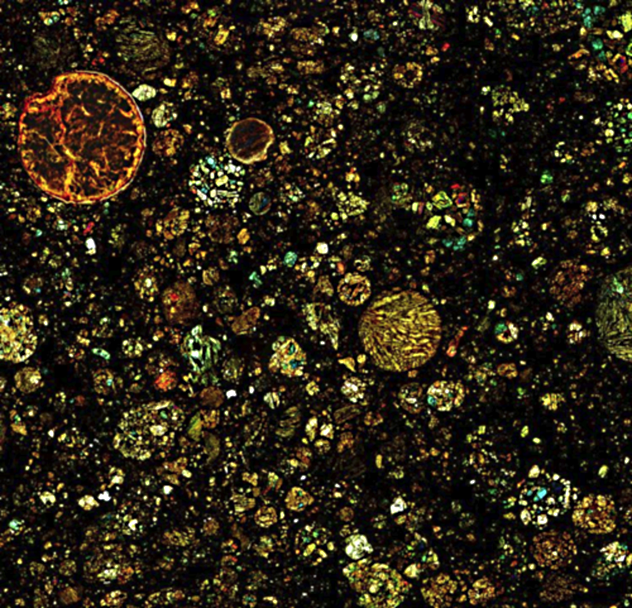
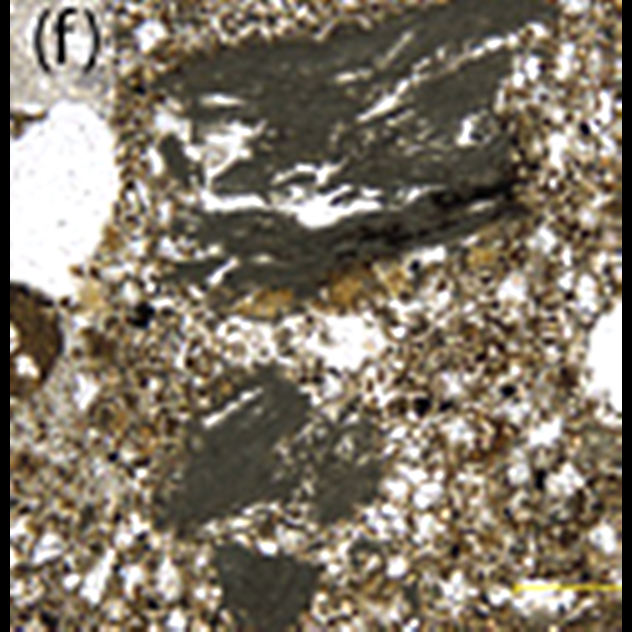
10 A Freeze-Frame Of The Solar System’s Formation
This is a thin section of a four-and-a-half-billion-year-old meteorite. The round blobs, called chondrules, are why these meteorites are called chondrites. Today, chondrites show scientists exactly how Earth and the rest of the solar system formed. Chondrites are literally older than dirt. They formed when the solar system was just a cloud of interstellar dust, some of which melted into chondrules. The rest of it started clumping together into bigger and bigger objects with more and more gravity. This became a runaway process that ended when the cloud’s center lit up as a star—our Sun. What remained of the dust and chondrules became planets, moons, asteroids, and comets. After that, all planets and most moons were big enough to continue developing on their own. None of their original material is left for scientists to study today, which is why chondrites like the one shown above are so important. Asteroids and a few other objects were too small to keep developing and simply lingered in the solar system for billions of years, occasionally breaking up and falling to Earth. Now scientists know that the bright chondrules shown above are embedded in material from the original interstellar dust cloud, which appears black in the above picture, caught in the act of forming a whole solar system.
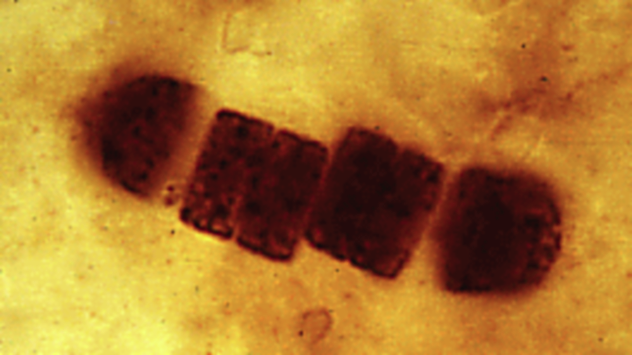
9 Possible Building Blocks For Life In Space
This blurry, seemingly out-of-focus image is the real-life equivalent of those chemical formulas you’ve seen in textbooks. It was taken with an instrument with an awesome name—“noncontact atomic force microscope”—and shows carbon and hydrogen atoms bonding together in three benzene rings. Astrobiologists love the six-sided benzene ring structure because it can be shaped into many different kinds of molecules that are likely to be found in space, particularly polycyclic aromatic hydrocarbons (PAHs). These and other carbon-based organic molecules make up about half of the dust and gas clouds that are floating between the stars. Since life on Earth is also carbon-based, one wonders if it originally came from those interstellar organic molecules. No one knows for sure, but NASA researchers have made an exciting discovery while studying PAHs. They exposed pyrimidine, a material that resembles PAHs, to conditions in the lab that mimic the harsh environment of space. The result: formation of uracil, cytosine, and thymine, three materials found in the genetic material of all life on Earth. Someday experts will figure out how life on Earth began. What we do know is that once it began, life suffered a series of mass extinctions. Possibly the worst extinction event ever was triggered by a very little creature named . . .
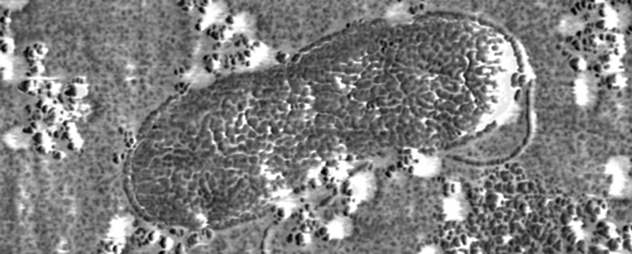
8 Cyanobacteria: The Cells That First Gave Earth Oxygen
This picture is exactly what it looks like: a bunch of bacterial cells seen through a microscope. This creature used to be known as blue-green algae but now goes by the name of cyanobacteria. The first amazing thing about these cells is that they are one billion years old. Scientists dug them out of billion-year-old geologic formations in Australia, where 29 other species have also been found. How can bacteria leave fossils? Cyanobacteria are bigger than most bacteria and have thick cell walls. They live in mats that build up into layered structures called stromatolites and oncolites. Ancient stromatolites, when cut in extremely thin slices, sometimes reveal fossilized cyanobacteria like the ones in this micrograph. An even more astonishing fact is that without those cyanobacteria in the image and many others like them, life as we know it would not exist today. In its youth, Earth’s atmosphere resembled the smoggy air on Saturn’s moon Titan. It was toxic to modern life, but some microbes, including cyanobacteria, could handle it. Then, about 2.3 billion years ago, cyanobacteria developed the ability to live off of sunlight through photosynthesis. One side effect of photosynthesis is oxygen, which was deadly to microbes that preferred smog. Since there were vast numbers of cyanobacteria, the Great Oxygenation Event changed the planet’s atmosphere and probably caused Earth’s greatest mass extinction. However, it also set the stage for today’s animals and plants. Right now it’s only guessed that cyanobacteria killed off the smog creatures, but we do know that there was once an event called the Great Dying, in which almost all of Earth’s life perished. One cause for that mass extinction was . . .
7 The Siberian Traps
This is what geologists call a thin section, because it’s, well, a very thin slice of rock. When you look at it under a microscope using polarized light, different minerals can be identified by color. (Also, thin sections make great rock art!) This is a thin section of leucocratic gabbro. The white part of the picture is the mineral plagioclase, and the blue one is amphibole. Note how the minerals are all clumped together; they’re apparently caught in a flow of black material that we can imagine rolling sluggishly, like Hawaiian lava, from left to right in this image. This actually once was runny, Hawaiian-style lava, and it began pouring out of the ground in what’s now Siberia one day roughly 250 million years ago. The flooding of the Siberian Traps happened during the Permian Period at the same general time as Earth’s biggest known mass extinction. The basalt flood lasted one million years. That’s a lot of lava—geologists estimate it would bury Europe to a depth of over 1 kilometer (0.6 mi). It wasn’t good news for life on Earth. While other factors were probably involved in the Great Dying, fumes and ash from this eruption blocked sunlight, and poisonous gases escaped from the lava to pollute both air and sea. During this time, an estimated 93–97 percent of all life vanished. Some say the flood was caused by a mantle plume; others think it was related to plate tectonics. The Siberian lava doesn’t say; its once-deadly crystals just sit there and shimmer at us. Earth goes through cycles of life and death. Some of it is recorded in rocks, but the atmosphere leaves no record. Or does it?
6 Earth’s Atmosphere 420,000 Years Ago
Those tiny bubbles of air aren’t rising in water. They’re frozen in ice that’s hundreds of thousands of years old. Analysis of the air tells scientists a lot about Earth’s ancient climate, how it has altered over time, and how it might change in the future. So how does air get into the ice, and how can it be dated? Snow crystals trap air as they fall to Earth. If the snow doesn’t melt, it turns into glacial ice with air bubbles. Everything stays in the same vertical position relative to everything else. Glaciers sometimes move horizontally, flowing over the land, but their interiors remain stable. Therefore, scientists can tell how old different horizontal glacier layers are even without carbon dating—the youngest layers are always on top. That’s how experts know that bubbles like these, found in ice cores from Antarctica and Greenland, contain air that’s as much as 420,000 years old. Changes in the amount of carbon dioxide in the air can certainly affect climate. That’s a big concern today, but fortunately, a little sea creature is helping us deal with it.
5 A Major Carbon Recycler
That’s not a satellite image of a forest with a road around it. It’s a microscopic view of Alteromonas, a recently discovered bacterium that plays a big role in keeping carbon dioxide (CO2) under control. Carbon exists everywhere on Earth. It’s present in the air in a delicate balance that the planet’s oceans help control. Seawater both absorbs and releases atmospheric CO2. Plankton eat the carbon that’s absorbed. When they die, their bodies sink into the lower depths of the ocean, where bacteria eat them. These bacteria then release CO2, which eventually goes back into Earth’s atmosphere. At least that’s what scientists think is going on. Most of the process happens miles under the ocean, where researchers can’t observe it. It was once believed that many different bacteria are involved. However, it was recently discovered that a single Alteromonas strain eats as much as an entire community of other organisms. The discovery makes it a lot easier for scientists to create models of the ocean’s carbon cycling. All they have to do is base their calculations on the Fat Albert of the sea.
4 Nine-Million-Year-Old Plants
Plants help keep the atmosphere breathable. The above pieces were flash-fossilized during a meteorite impact millions of years ago. Scientists had no idea organic matter could withstand so much heat. Thanks to this discovery, we now know it’s possible that life on Mars, if it ever existed, may have been preserved the same way. Here’s what happened: A series of seven different space objects crashed into what’s now Argentina, with the last impact occurring about nine million years ago. The ground there was covered by a powdery soil called loess, which melted and turned into glass very quickly. Experts did a series of tests; after many crispy failures, they discovered that at temperatures over 1,480 degrees Celsius (2,700 °F), the water in a plant’s outside layers absorbs enough heat to protect the delicate inner structures. Something similar happens when you deep-fry food. Mars is also covered in loess and has a lot of impact craters. It hasn’t had rivers and oceans for billions of years, but it once did. Life could have existed there, and it’s quite possible that ancient Martian life might have been preserved in impact glass, just like these Earth plants were.
3 A Freeze-Frame Of The World’s Largest Recent Volcanic Eruption
This may look like a closeup of Van Gogh’s Starry Night, but it’s really another geologic thin section of volcanic rock. There is no smearing together here but lots of sharp edges instead. This was a violent eruption, not a runny, Hawaiian-style flow. Those larger chunks are clasts—broken mineral fragments. They’re embedded in pulverized rock that flows around them. Look closely, and you’ll see dark voids in the powdered stone that stretch out like pulled, hot taffy. This is a small piece of the Toba supereruption from some 75,000 years ago. It was Earth’s biggest known eruption during human history, blasting 2,900 cubic kilometers (700 mi3) of magma and three trillion kilograms (6.6 trillion lb) of sulfur into the sky. Mineral crystals were shattered into clasts as they exploded out of the vent. Seconds later, they were embedded in hot, gassy volcanic ash. The gas quickly dissipated, leaving behind spaces in the ash particles that look black under polarized light. Tens of thousands of years later, geologists studying that debris are still awed by Toba’s violence. Ash from the eruption fell as far as Eastern Africa, 7,000 kilometers (4,300 mi) away.
2 Humans Taming Fire
This one is exactly what it looks like. The tan stuff is dirt, the lighter particles are ash from a wood fire, and the dark gray material is plant matter that has been partially burned. What’s amazing is that it proves people had fire under their control one million years ago—far earlier than anyone expected. Estimates of exactly when humans tamed fire have always been iffy. It’s hard to tell whether layers of ancient ash were left by a wildfire or a cooking fire. A few years ago, scientists used advanced techniques on ashes, including those shown above. The ash came from a one-million-year-old fire found in a South African cave. It was undisturbed and couldn’t have been caused by natural processes. Stone tools were found nearby. What we see here are the ashy remains of a plant that somebody, possibly Homo erectus, carried into that cave one million years ago. They probably weren’t vegetarians because burned bones were found, too. The control of fire was our biggest step toward becoming the masters of Earth that we are today. But are we really masters? Scientists are beginning to realize that the largest mass of living organisms on Earth may actually dwell in the rocky crust under the oceans. These tiny creatures are called . . .
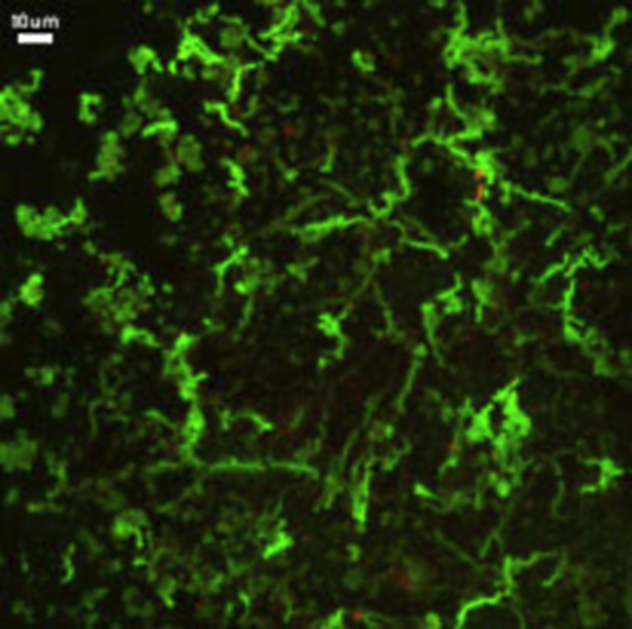
1 Endoliths
It would be easiest to let the scientists tell you what these pretty green things are: “Twisted mineral stalks produced by iron oxidizing bacteria recovered from mineral incubation experiments in Juan de Fuca boreholes.” The operative word here is “boreholes.” Scientists drilled down into the seafloor and found bacteria living there. These little rock-dwellers, called endoliths, have been covered before. They live in rock and eat it. Scientists have known about them for years, but only now is it starting to sink in just how many endoliths there could be on Earth. Most of Earth is covered by oceanic crust. This seafloor is made of basalt lava that erupts at mid-ocean ridges and then moves away from the ridges on a sort of geological conveyor belt. There’s plenty of water and heat available—both things that are necessary for life on Earth. Furthermore, aquatic life already thrives on the mid-ocean ridges at hydrothermal vents. Why shouldn’t life do just as well inside the seafloor? Now, imagine all of that oceanic crust being inhabited. The scientists who took this image of green endolith stalks believe that it indeed could be a great home for such a life-form. Others even believe that that the seafloor may contain more biomass than land and marine life combined! Barb blogs about Earth science and the American Civil War at http://bjdeming.com. She also just published fiction about the meeting of a Buddhist monk and a US soldier on a Vietnam battlefield in 1967 and currently is working on a book about the very first cat.